Part 25: Results - Round 7
14: LP Isomers
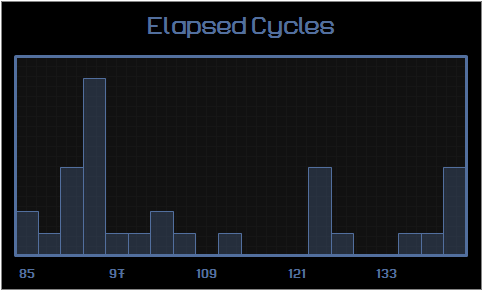
Cycles Reactor Symbols
gggol 85 1 36
CannibalK9 85 1 36
TheKnife 86 1 34
Pseudodude 90 1 35
Serbaldrig 90 1 35
ZndyMinner 90 1 35
Krackor 90 1 35
Nethris 92 1 22
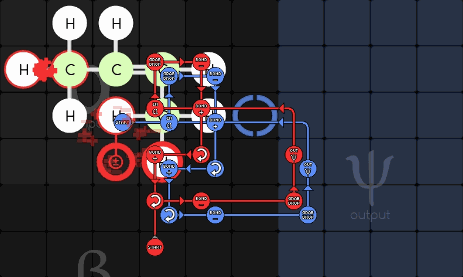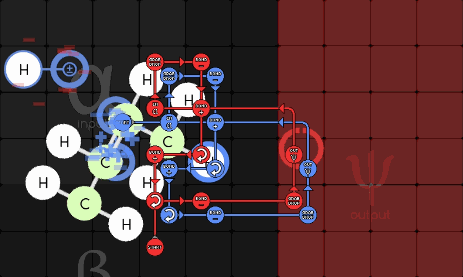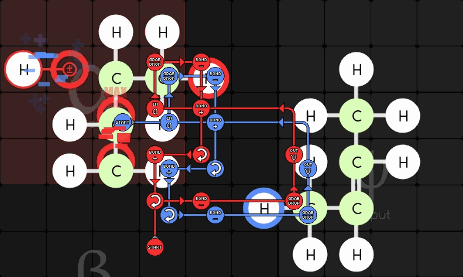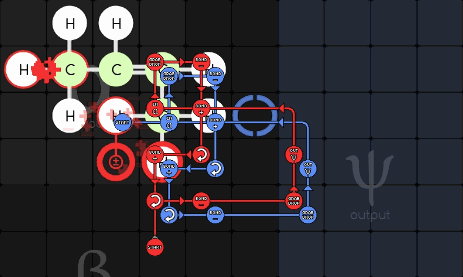
gggol/CannibalK9 85-1-36
15: Natural Chemo
Cyclces Reactor Symbols
Pseudodude 215 1 16
Leylite 236 1 16
Serbaldrig 144 1 17
Krackor 144 1 17
Nethris 243 1 17
Carlbunk 248 1 17
Leveling 326 1 18
ZndyMinner 397 1 18
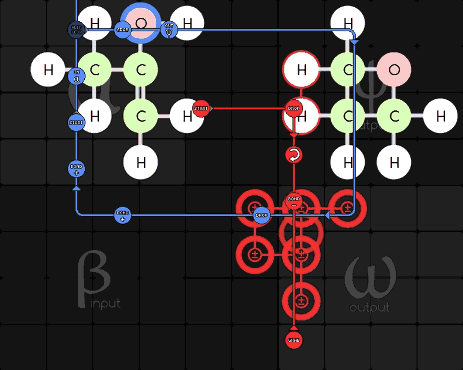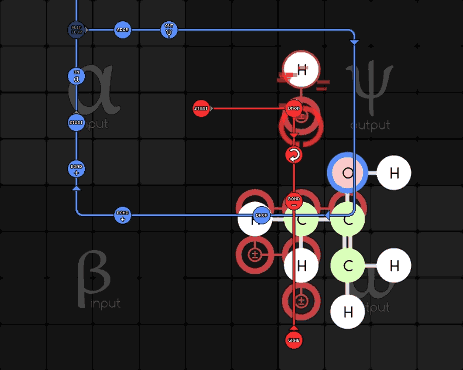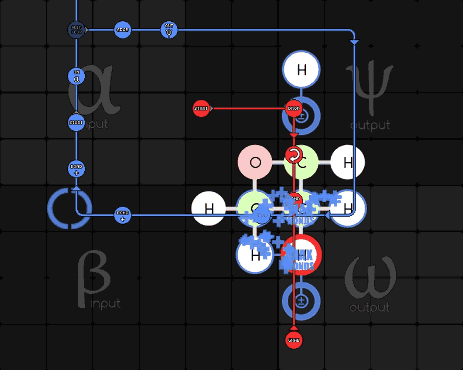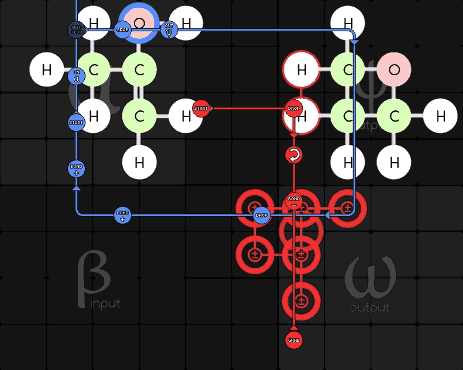
Pseudodude 215-1-16
Challenge 7: The Chem in SpaceChem
Cycles Reactor Symbols
Jabor 216 1 21
Carlbunk 292 1 21
gggol 153 1 22
ecco2 282 1 22
GuavaMoment 343 1 23
Serbaldrig 365 1 23
CannibalK9 238 1 24
Pseudodude 639 1 24
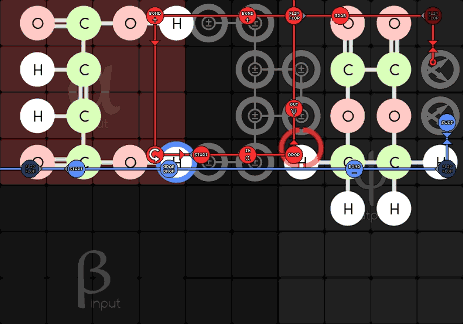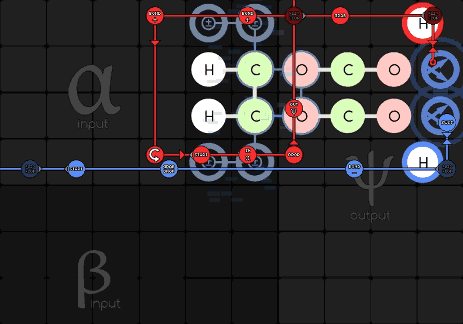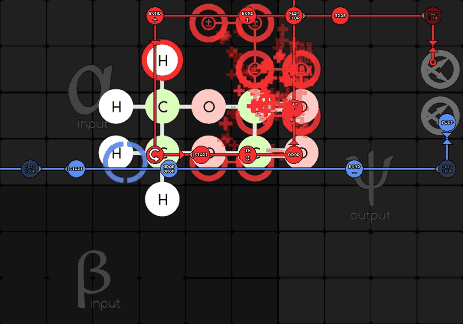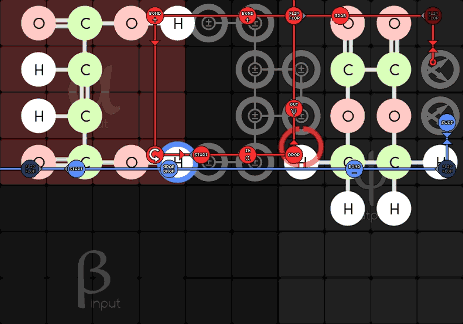
Jabor 216-1-21
Overall Standings - Upper Tier
Pseudodude 32
Serbaldrig 25 (Fewer participation points)
Carlbunk 25
CannibalK9 24
ecco2 23
gggol 21
GuavaMoment 20
MrBlarney 19
Everyone Else <=18
Overall Standings - Lower Tier (Not including upper tier placements)
Leylite 52
cearn 50 (Fewer participation points)
ToughThought 50
DariusRaider 48
Krackor 41
Jabor 39
TheKnife 38
Nethris 37 (Fewer participation points than Leveling)
Everyone Else <=37
---
As promised, that pile of stuff from cearn.
cearn posted:
My previous job happens to be in chemical technology, particularly modelling EthyleneOxide (C2H4O) reactors.
Ethylene Oxide (EO for short) is a widely used raw material, particularly to make glycols. These are then used for things like anti-freeze, coolants, solvents and a host of other things. To create EO from basic ethylene plus oxygen, you have to consider three separate reactions:
Reaction 1, the epoxidation reaction, is the one you want. The other two are burning reactions and are undesired. Normally reaction 2 dominates (boo!), but with the right catalyst you can switch the balance to reaction 1 (yay!).
- C2H4 + ½ O2 → C2H4O
- C2H4 + 3 O2 → 2 CO2 + 2 H2O
- C2H4O + 2½ O2 → 2 CO2 + 2 H2O
The reactions all take place in a vat of ~10,000 13m long tubes. A mixture of gases goes in, and a mixture of different gases comes out. The reactions run around temperatures of 520 K, and because the reactions are highly exothermic, you have to send in the gas at a lower temperature (~ 400 K), heat it up to the right temperature range, and keep it at that temperature by cooling the tubes from the outside so that the reactor doesn't overheat and blow up.
One of the most important quantities in this process is the selectivity. This is the ratio of created C2H4O and the used up C2H4:
It's basically an efficiency measure, telling you how much C2H4 went into reaction 1, compared to reactions 2 and 3. At the right temperature range, the selectivity can be around 90%. And because even 0.1% can mean several million dollars / year, getting to that temperature range as soon as possible is rather important. Even decreasing the heat-up zone by a few centimeters can make a world of difference.
- S = ΔC2H4O / ΔC2H4
One last thing: if you do the stoichiometry, you'll see that there are actually 6 ways that you can express the selectivity. These should of course all give the same result, but in the real world where you have errors in your composition measurements, the molecular balance can be a little off, and those 6 selectivity values can differ by as much as 30% (remember: 0.1% was already important). Fortunately, there are mathematical trickeries you can employ to balance all of it out again. But that's another story.